In November 2020, we published an article outlining the blood biomarker study coming out of the National Institutes of Health Clinical Center in Bethesda, Maryland, with significant implications for detecting, classifying, and treating traumatic brain injury (TBI) at all severity levels.[1] The study supported ongoing advances in the use of blood-based biomarkers to assist in immediate detection and diagnosis of concussion. The study also has far reaching implications on planning a recovery path for individuals with traumatic brain injury, and to assist with potential legal claims involving concussion injuries. The study has particularly high promise because the current clinical tools used to diagnose concussion, such as Glasgow Coma Scale (GCS) and imaging studies, are not always reliable indicators of TBI, particularly for patients with mild TBI (mTBI) that often cannot be detected by conventional neuroimaging such as MRI or CT alone.
In follow up to that recent article, the American Association for Clinical Chemistry (AACC) has announced that the Food and Drug Administration has approved the use of two well-studied protein biomarkers[2] for concussion injury: glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase -L1(UCH-L1), both from a single manufacturer approved for in vitro diagnosis use in the US.
The AACC cautions that the use of biomarkers remains limited in ruling out concussion. While concussion biomarkers do generally have an excellent diagnostic ability to correctly identify patients with concussions, they also do poorly in identifying the percentage of patients without concussion compared to CT scans. As such, the AACC recommends that patients with a positive biomarker test result still undergo a CT scan to eliminate false positives.[3]
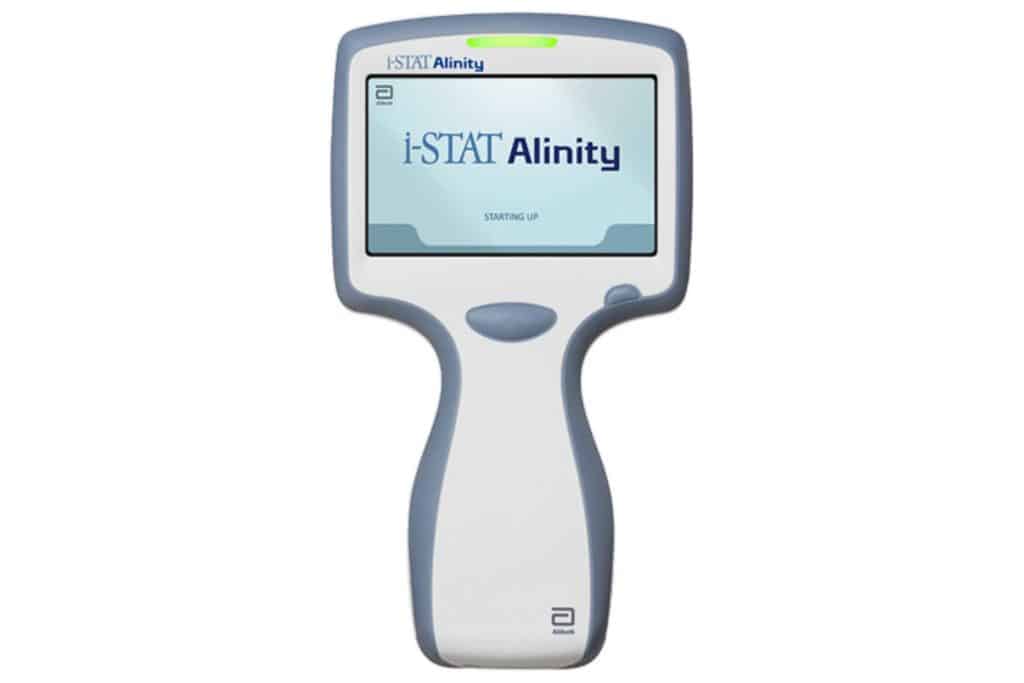
In January 2021, the FDA approved the first rapid handheld blood test for suspected mTBI. The test gives results within 15 minutes. Manufactured by Abbott Laboratories, the i-STAT alinity TBI plasma test measures the GFAP and UCH-L1 proteins in the plasma of the patient’s blood. Abbott Laboratories stated in a press release that elevated concentrations of these two biomarkers closely correlate to brain injury, with 95.8% sensitivity and greater than 99% ability to predict patients without brain injury. Abbott developed the rapid handheld blood test in collaboration with the U.S. Department of Defense.[4]
This test requires a small blood sample, drawn from the arm. Plasma is extracted from the sample with a centrifuge, applied to the test’s cartridge, and is then inserted into the handheld device.
Adler Giersch remains committed to monitoring advances in research and science to ensure that our clients have access to the best care possible, and to assist with accessing justice for victims of traumatic brain injury. We will continue to monitor the availability of this tool in practice and report on its capability as more information becomes available.
[1] https://www.adlergiersch.com/provider-blog/new-study-confirms-blood-biomarker-can-detect-traumatic-brain-injury/
[2] With biomarkers, scientists look for low abundance proteins found in tissue, blood, urine and other body fluids which may provide vital early indicators of disease.
[3] https://www.aacc.org/cln/articles/2020/september/concussion-biomarkers-where-they-stand-now#:~:text=Most%20concussion%20biomarker%20tests%20are,baseline%20within%20minutes%20to%20hours.
[4] https://abbott.mediaroom.com/2021-01-11-Abbott-Receives-FDA-510-k-Clearance-for-the-First-Rapid-Handheld-Blood-Test-for-Concussions
